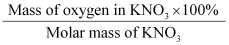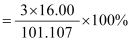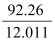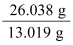5 Mole Concept and Molar Masses
Mole Concept and Molar Masses
- 1 mole of any substance can be defined as:
- Amount of a substance that contains as many particles (atoms, molecules or ions) as there are atoms in 12 g of the 12C isotope
- Avogadro number or Avogadro constant (NA); equal to 6.022 × 1023 particles
- Example − 1 mole of oxygen atoms = 6.022 × 1023 atoms
1 mole of carbon dioxide molecules = 6.022 × 1023 molecules
1 mole of sodium chloride = 6.022 × 1023 formula units of sodium chloride
Molar mass of a substance can be defined as:
- Mass of one mole of a substance in grams
- Numerically equal to atomic/molecular/formula mass in u.
- Example − Molar mass of CO2 = 44.011 g mol−1
Molar mass of NaCl = 58.5 g mol−1
| Examples
1. What number of moles contains 3.011 × 1023 molecules of glucose? Solution: 1 mole of glucose is equivalent to 6.022 × 1023 molecules of glucose. Hence, 3.011 × 1023 molecules of glucose will be present in
Thus, 0.5 mole of glucose contains 3.011 × 1023 molecules of glucose. 2. What is the mass of a mole of fluorine molecule? Solution: 1 mole of fluorine molecule contains 6.022 × 1023 molecules and weighs 38 g. Therefore, mass of a fluorine molecule = = 6.31 × 10−23 g |
Percentage Composition
Mass percent of an element = 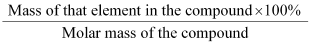
| Example
What is the mass percent of oxygen in potassium nitrate? (Atomic mass of K = 39.10 u, atomic mass of N = 14.007 u, atomic mass of O = 16.00 u) Solution: Atomic mass of K = 39.10 u (Given) Atomic mass of N = 14.007 u (Given) Atomic mass of O = 16.00 u (Given) Therefore, molar mass of potassium nitrate (KNO3) = 39.10 + 14.007 + 3(16.00) = 101.107 g Therefore, mass percent of oxygen in KNO3 = = = 47.47% (approx) |
- Empirical formula and molecular formula:
| Empirical formula | Molecular formula |
| Represents the simplest whole number ratio of various atoms present in a compound | Represents the exact number of different types of atoms present in a molecule of a compound |
- Empirical formula is determined if mass % of various elements are known.
- Molecular formula is determined from empirical formula if molar mass is known.
| Example
A compound contains 92.26% carbon and 7.74% hydrogen. If the molar mass of the compound is 26.038 g mol−1, then what are its empirical and molecular formulae? Solution: Mass percent of carbon (C) = 92.26% (Given) Mass percent of hydrogen (H) = 7.74% (Given) Number of moles of carbon present in the compound = = 7.68 mol Number of moles of hydrogen present in the compound = 7.68 mol Thus, in the given compound, carbon and hydrogen are present in the ratio C : H = 7.68 : 7.68 = 1 : 1 Therefore, the empirical formula of the compound is CH. Empirical formula mass of CH = (12.011 + 1.008)g = 13.019 g Molar mass of the compound = 26.038 g (Given) Therefore, n = = = 2 Hence, the molecular mass of the compound is (CH)n, i.e., (CH)2 or C2H2. |
| Interconversion among number of moles, mass and number of molecules |
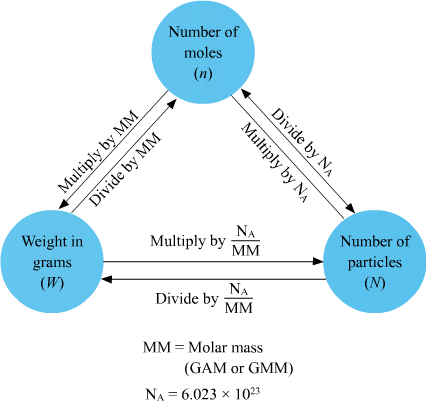 |

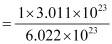 mol = 0.5 mol (of glucose)
mol = 0.5 mol (of glucose)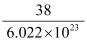 g
g