3 Vapour Pressure of Liquid Solutions
- Let p1, p2 = Partial vapour pressure of two volatile components 1 and 2 of a mixture
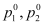 = Vapour pressure of pure components 1 and 2
= Vapour pressure of pure components 1 and 2
x1, x2 = Mole fractions of the components 1 and 2
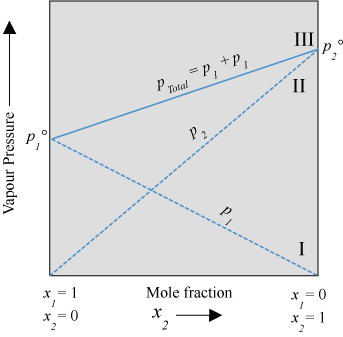
ptotal = Total vapour pressure of the mixture
Raoult’s law
For a solution of volatile liquids, the partial vapour pressure of each component in the solution is directly proportional to its mole fraction.
That is, for component 1,
p1∝ x1
And, 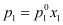
For component 2,
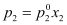
According to Dalton’s law of partial pressures,
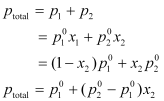
From the above equation, it can be concluded that:
-
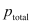 can be related to mole fraction of any one component.
can be related to mole fraction of any one component.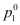 varies linearly with x2.
varies linearly with x2.- Depending upon
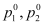 and
and 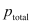 increases or decreases with the increase of x1.
increases or decreases with the increase of x1.
The plot of vapour pressure and mole fraction of an ideal solution at constant temperature is shown below.
here, Maximum value of 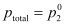
Minimum value of 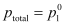
Here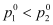 , because it is assumed that component 1 is less volatile than component 2.
, because it is assumed that component 1 is less volatile than component 2.
The component of vapour phase in equilibrium with the solution is determined by the partial pressures of the components.
Let y1, y2 = Mole fractions of the components 1 and 2 respectively in the vapour phase
According to Dalton’s law of partial pressures,
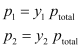
In general, we can write
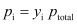
Raoult’s Law as a Special Case of Henry’s Law
-
- According to Raoult’s law, the vapour pressure of a volatile component in a given solution is
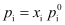
- According to Henry’s law, the partial vapour pressure of a gas (the component is so volatile that it exists as gas) in a liquid is p = KH x
- According to Raoult’s law, the vapour pressure of a volatile component in a given solution is
-
- It can be observed that in both the equations, the partial vapour pressure of the volatile component varies directly with its mole fraction. Only the proportionality constants KH and are different. Thus, Raoult’s law becomes a special case of Henry’s law in which KH is equal to .
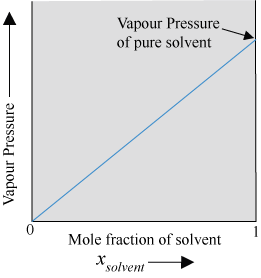
Vapour Pressure of Solutions of Solids in Liquids
-
- When a non-volatile solute is added to a solvent, the vapour pressure of the liquid decreases.
Reason: The number of solvent molecules on the surface decreases and as a result, number of solvent molecules escaping from the surface decreases.
-
- Raoult’s law in general form: For any solution, the partial vapour pressure of each volatile component in the solution is directly proportional to its mole fraction.
- Let us take a binary solution made by dissolving a non-volatile solute in a solvent. Since the solute is non-volatile, only the solvent molecules contribute to vapour pressure.
Let 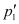 = Vapour pressure of the solvent
= Vapour pressure of the solvent
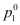 = Vapour pressure of the solvent in pure state
= Vapour pressure of the solvent in pure state
x1 = Mole fraction of the solvent
Then, according to Raoult’s law,
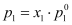
Here, the proportionality constant is equal to the vapour pressure of the solvent in pure state.
The plot of vapour pressure vs. mole fraction of the solvent, which is linear, is shown below.