4 Ideal and Non-Ideal Solutions
Ideal Solution
- Solutions which obey Raoult’s law over the entire range of concentrations
- For ideal solution:
- Enthalpy of mixing of the pure components to form the solution, Δmix H = 0
- Volume of mixing, Δmix V = 0
- An ideal solution will be formed when intermolecular forces of attraction between the molecules of solute (A − A) and those between the molecules of solvent (B − B) are nearly equal to those between solute and solvent molecules (A − B).
Examples: n-Hexane and n-heptane, bromoethane and chloroethane, benzene and toluene
Non-Ideal Solutions
- Solutions which do not obey Raoult’s law over the entire range of concentration
- The vapour pressure of a non-ideal solution is either higher or lower than that predicted by Raoult’s law.
- Positive deviation from Raoult’s law − When vapour pressure of solution is higher
- Negative deviation from Raoult’s law − When vapour pressure of solution is lower
- Solution showing positive deviation from Raoult’s law
- The plot of vapour pressure of two component solutions as a function of mole fraction is shown below.
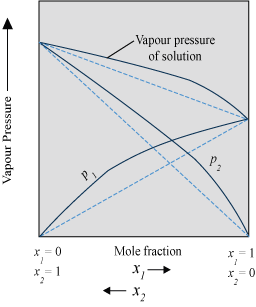
- The intermolecular forces of attraction between solute-solvent molecules are weaker than those between solute-solute molecules and solvent-solvent molecules. Therefore, solvent molecules can easily escape, resulting in increase in vapour pressure.
Example: Ethanol and acetone mixture.
- Solution showing negative deviation from Raoult’s law
- The plot of vapour pressure of two component solutions as a function of mole fraction is shown below.
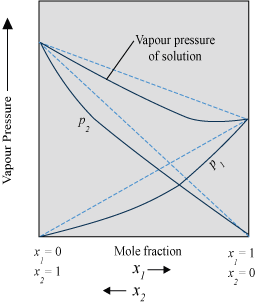
- The intermolecular forces of attraction between solute-solute molecules and solvent-solvent molecules are weaker than those between solute-solvent molecules. This results in the decreasing of vapour pressure.
Example − Chloroform and acetone mixture
The intermolecular attractive forces between solute-solvent molecules increase due to the formation of H-bond.
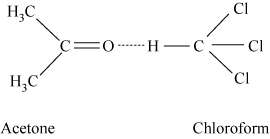
Azeotropes
- Binary mixtures which have the same composition in liquid and vapour phase, and have constant boiling points
- Not possible to separate the components by fractional distillation
- Two types − (a) Minimum boiling azeotrope (b) Maximum boiling azeotrope
Minimum boiling azeotrope − Solution showing a large positive deviation from Raoult’s law of specific composition
Example: Ethanol-water mixture containing ethanol approximately 95% by volume
Maximum boiling azeotrope − Solution showing a large negative deviation from Raoult’s law at specific composition
Example: Nitric acid-water mixture containing 68% nitric acid and 32% water by mass
The boiling point of this azeotrope is 393.5 K.
