5 Colligative Properties and Determination of Molar mass
- Properties which depend upon the number of solute particles present in the solution irrespective of their nature, relative to the total number of particles present in the solution
- Examples: Relative lowering of vapour pressure of the solvent, depression of freezing point of the solvent, elevation of boiling point of the solvent, osmotic pressure of the solution
Relative Lowering of Vapour Pressure
- Relative lowering of vapour pressure is equal to the mole fraction of the solute.
i.e., 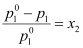
Where,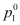 = Vapour pressure of the solvent
= Vapour pressure of the solvent
p1 = Vapour pressure of the solution
x2 = Mole fraction of the solute
However,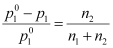
Where, n2 = Number of moles of solute
n1 = Number of moles of solvent
Now, 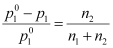
For dilute solution, n1 >> n2
Therefore, 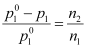
Or, 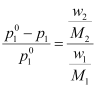
Or, 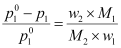
Where, w2 = Mass of solute
M2 = Molar mass of solute
w1 = Mass of solvent
M1= Molar mass of solvent
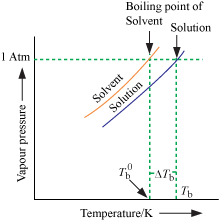
Elevation of Boiling Point
- With addition of non-volatile solute, vapour pressure decreases and hence, boiling point increases.
Here, 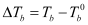 = Elevation of boiling point
= Elevation of boiling point
Where,
Tb = Boiling of solution
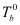 = Boiling point of solvent
= Boiling point of solvent
ΔTb ∝ m (for dilute solution)
⇒ ΔTb = Kbm
Where, m = Molality
Kb = Proportionality constant known as Boiling Point Elevation Constant or Molal Elevation Constant or Ebullioscopic constant (Unit = K kg mol−1)
However, 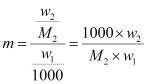
Where, w2 = Mass of solute
M2 = Molar mass of solute
w1 = Mass of solvent
Now, 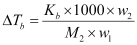
Or, 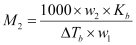
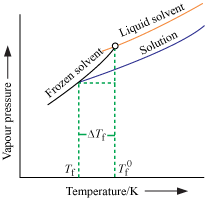
Depression of Freezing point
- With addition of non-volatile solute, vapour pressure decreases, which leads to a decrease in freezing point.
Here, 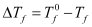 = Depression of freezing point
= Depression of freezing point
Where, 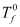 = Freezing point of solvent
= Freezing point of solvent
Tf = Freezing point of solution
For dilute solution (ideal solution),
ΔTf ∝ m
⇒ ΔTf = Kf m
Where, m = Molality
Kf = Proportionality constant known as Freezing Point Constant or Molal Depression Constant or Cryoscopic constant (Unit = K kg mol−1)
However, 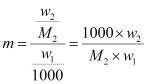
Where, w2 = Mass of solute
M2 = Molar mass of solute
w1 = Mass of solvent
Now, 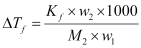
Or, 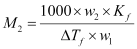
The value of Kb and Kf can be determined by the following relations:
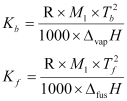
Where, M1 = Molar mass of the solvent
R = Gas constant
ΔvapH = Enthalpy of vaporisation
ΔfusH = Enthalpy of fusion
Osmosis and Osmotic Pressure
- The process of flow of solvent molecules from pure solvent to solution or from solution of lower concentration to solution of higher concentration through a semi-permeable membrane is called osmosis.
- The pressure required to just stop the flow of solvent due to osmosis is called osmotic pressure (π) of the solution.
- The osmotic pressureπ has to be applied to the solution to prevent osmosis.
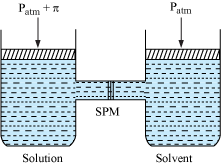
- For dilute solution, osmotic pressure is directly proportional to the molarity C of the solution at a given temperature T.
That is, π = CRT (R = Gas constant)
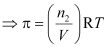
Where, n2 = Number of moles of solute
V= Volume of the solution in litres
Again, 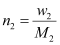
Where, w2 = Mass of the solute
M2 = Molar mass of the solute
Now, 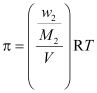
Or, 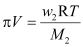
Or, 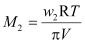
Now, osmosis does not occur between two isotonic solutions.
Isotonic solutions − Solutions having same concentration of solute These solutions have same osmotic pressure.
Hypotonic solution − Solution having a low concentration of solute relative to another.
Hypertonic solution − Solution having a high concentration of solute relative to another.
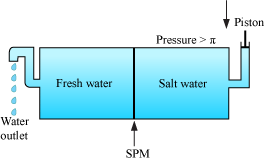
Reverse Osmosis and Water purification
- If the pressure applied on the solution is greater than its osmotic pressure, then the direction of osmosis is reversed i.e., the solvent starts passing from solution to solvent. The phenomenon is called reverse osmosis.
- This phenomenon is used in purification (desalination) of sea water.