6 Abnormal Molar Masses
Abnormal Molar Masses
- Colligative property ∝
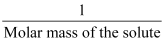
- Due to association or dissociation of molecules, the molar mass of a substance calculated from its colligative property is either lower or higher than the expected or normal value. Such molar mass is called abnormal molar mass.
Dissociation:
KCl 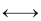 K+ + Cl−
K+ + Cl−
Association:
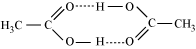
2CH3COOH 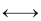 (CH3COOH)2
(CH3COOH)2
- To account for the extent of dissociation or association, van’t Hoff introduced a factor i, known as the Van’t Hoff factor.

Value of i
For association, i < 1
For dissociation, i > 1
No association or dissociation, i = 1
- Modified equations for colligative properties after inclusion of van’t Hoff factor
- Relative lowering of vapour pressure of solvent,
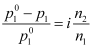
- Elevation of boiling point, ΔTb = iKb m
- Depression of freezing point, ΔTf = iKf m
- Osmotic pressure of solution, π = in2RT/V
