Chapter 1 Matter In Our Surroundings Note
Matter in Our Surroundings
Matter
- All things which we see around us.
- Use in our everyday life together constitute matter.
- Anything which occupies space and has mass is called matter.
- Matter is made up of particles.
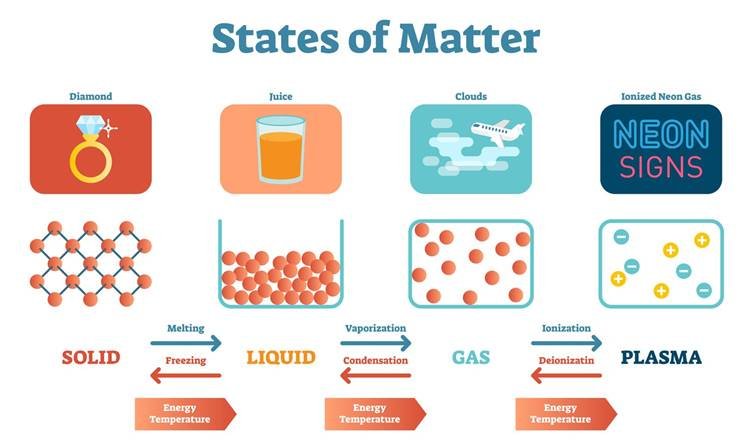
States of Matter
- Matter can be classified on the basis of interparticle forces and the arrangement of particles.
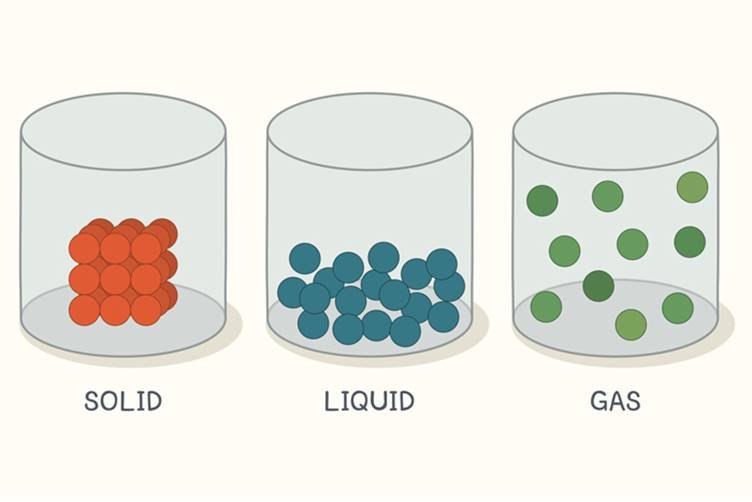
- Solid
- Liquid
- Gas
- These three forms of matter are interconvertible by increasing or decreasing pressure and temperature.
- For example, ice can be converted from solid to a liquid by increasing the temperature.
| Solid | Liquid | Gas |
| Not Compressible | Not Compressible | Highly Compressible |
| High Density | High Density | Low Density |
| Definite Volume | Definite Volume | Not definite volume (fill container completely) |
| Retains its own shape | Obtain the shape of container | Obtain the shape of container |
| Motion limited to vibrational movement | Slow diffusion (particles can slip past each other) | Rapid diffusion |
| Low expansion on heating | Low expansion on heating | High expansion on heating |
Atomic view of the three states of matter
Evaporation
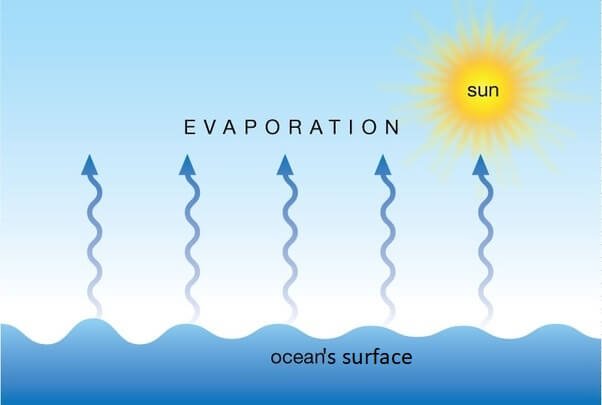
- The phenomenon by which molecules in liquid state undergo a spontaneous transition to the gaseous phase at any temperature below its boiling point is called evaporation.
- For example, the gradual drying of damp clothes is caused by the evaporation of water to water vapour.
Factors affecting evaporation
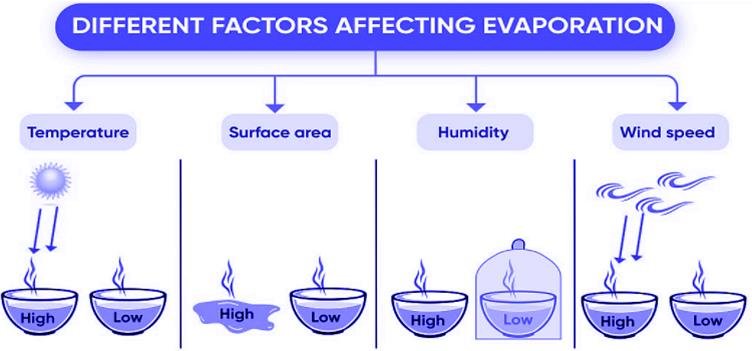
- Temperature: The rate of evaporation increases with an increase in temperature.
- Surface area: The rate of evaporation increases with an increase in surface area.
- Humidity: The rate of evaporation decreases with an increase in humidity.
- Wind speed: The rate of evaporation increases with an increase in wind speed.
Cooling due to evaporation
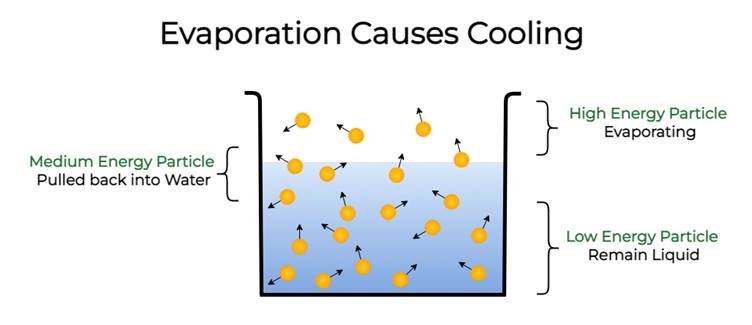
- During evaporation, the particles of a liquid absorb energy from the surroundings to overcome the inter-particle forces of attraction and undergo the phase change.
- The absorption of heat from the surrounding makes the surrounding cool.
- For example, sweating cools down our body.
Physical Nature of Matter
- A physical property is that aspect of the matter that can be observed or measured without changing its nature or composition.
- It is independent of the amount of matter present.
- Include appearance, colour, odour, density, texture, melting point, boiling point, solubility, etc.
Characteristics of Particles of Matter
- Matter is anything that has mass and occupies space.
- Everything that we can touch, see, hear, taste and also smell is matter.
- It is made up of really tiny particles which cannot be seen through the eye.
- The particles of which the matter is comprised influence its state and properties (physical and chemical).
- Particles of matter have spaces between them.
- This characteristic is one of the concepts behind the solubility of a substance in other substances.
- For example, on dissolving sugar in water, there is no rise in water level because the particles of sugar get into the interparticle spaces between the water particles.
- Particles of matter are always in motion-Particles of the matter show continuous random movements due to the kinetic energy they possess. A rise in temperature increases the kinetic energy of the particles, making them move more vigorously.
- Particles of matter attract each other
- In every substance, there is an interparticle force of attraction acting between the particles.
- To break a substance, we need to overcome this force.
- The strength of the force differs from one substance to another.
Diffusion
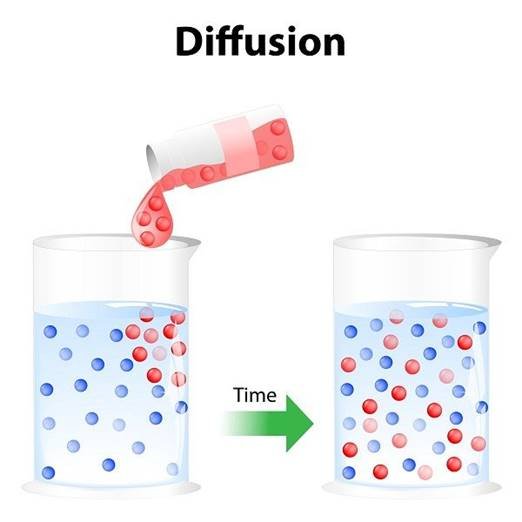
- When the particles of matter intermix on their own with each other, the phenomenon is called diffusion.
- For example: spreading of ink in water.
- During diffusion, the particles occupy the interparticle spaces.
- The rate of diffusion increases with increase in the temperature, due to increase in kinetic energy of the particles.
Effect of change of temperature on state of matter
- On increasing temperature, the kinetic energy of the particles of the matter increases and they begin to vibrate with a higher energy.
- The interparticle force of attraction between the particles reduces and particles get detached from their position and begin to move freely.
- As a result, the state of matter begins to change.
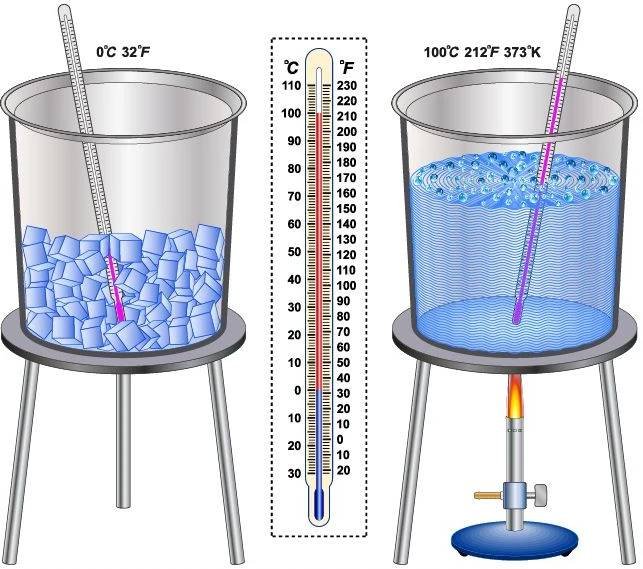
Melting point
- The melting point of a solid is defined as the temperature at which solid melts to become liquid at the atmospheric pressure.
- At melting point, these two phases, i.e., solid and liquid are in equilibrium, i.e., at this point both solid state and liquid state exist simultaneously.
Boiling point
- The boiling point of a liquid is defined as the temperature at which the vapour pressure of the liquid is equal to the atmospheric pressure.
Latent heat of vaporisation
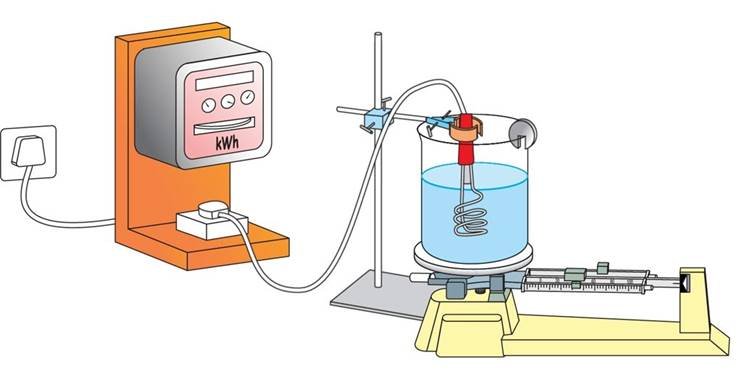
- It is the amount of heat energy that is required to change 1 kg of a liquid into gas at atmospheric pressure at its boiling point.
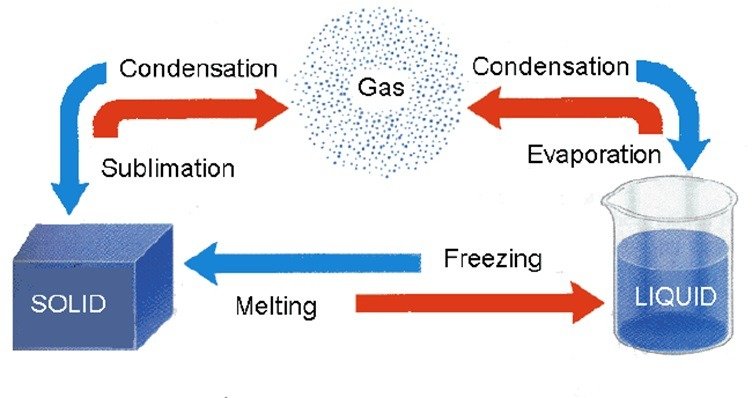
Sublimation

- The transition of a substance directly from its solid phase to gaseous phase without changing into the liquid phase (or vice versa) is called sublimation.
- Sublimation – Solid to Gas Phase Transformation
- The change of state of a substance directly from a solid to gas, without changing into the liquid state (or vice versa) is called sublimation.
- Example: camphor, naphthalene, ammonium chloride, solid carbon dioxide and iodine.
Types of Matter
- There are two ways in which matter can be classified-
- On the basis of its physical nature (physical state).
- On the basis of its chemical constitution.
Characteristics of Particles of Matter
- are very small.
- have spaces between them.
- are continuously moving.
- attract each other.
Diffusion
- Intermixing of particles of two different types of matter on their own is called diffusion.
- The rate of diffusion increases on increasing the temperature of the diffusing substance (by heating).
- Examples of diffusion in gases:
- The aroma of food being cooked in the kitchen reaches us even from a considerable distance due to diffusion.
- The fragrance of a perfume spreads due to the diffusion of the perfume particles into air.
- Examples of diffusion in liquids:
- The spreading of ink in water, on its own, is due to the diffusion of ink particles in the water.
- Examples of diffusion in solids:
- If two metal blocks are bound together tightly and kept undisturbed for a few years, then the particles of one metal are found to have diffused into the other metal.
TERMS
(a) Melting Point
- The temperature at which a solid melts to become a liquid, at atmospheric pressure, is called its melting point.
- Melting point is the characteristic property of a substance. For example, melting point of ice is 0 degree C (273 K).
(b) Freezing or Solidification
- The process, in which a liquid changes to its solid form, on cooling at a specific temperature, is called freezing or solidification.
(c) Latent heat:
- The hidden heat which breaks the force of attraction between the molecules is known as the latent heat.
- Since, the heat energy is hidden in the bulk of the matter, it is called latent heat.
(d) Latent heat of fusion:
- The heat energy required to convert 1 kilogram of a solid into liquid at atmospheric pressure, at its melting point, is known as the latent heat of fusion.
- When we supply heat energy to water, the particles start moving faster.
- At a certain temperature, a point is reached when the particles have enough energy to break free from the forces of attraction of each other.
- At this temperature, the liquid starts changing into a gas.
(e) Boiling Point: (Liquid → Gas)
- The temperature at which a liquid starts boiling, at atmospheric pressure, is called its boiling point.
- Boiling is a bulk phenomenon.
- Particles from the bulk of the liquid gain energy to change into the gaseous state.
- For example, boiling point of water is 1000C.
- (Or 1000C = 273 + 100 = 373 K)
(f) Latent heat of vapourisation:
- The heat energy required to convert 1 kilogram of liquid into gas, at atmospheric pressure, at its boiling point, is known as the latent heat of vapourisation.
(g) Condensation (Gas → Liquid)
- The process, in which a gas, on cooling, turns into a liquid at a specific temperature is called condensation or liquefaction.
- The heat removed from the surface through evaporation is released into the atmosphere by the formation of clouds.
- This process cools the Earth’s climate.
(h) Freezing point (Liquid → Solid)
- The temperature at which the state of a substance changes from a liquid to a solid is called the freezing point of that substance.
(i) Evaporation (Liquid à Gas)
- The process of conversion of a substance from the liquid state to the gaseous state at any temperature below its boiling point is called evaporation or vapourisation.
- Evaporation is a surface phenomenon.
Factors Affecting Evaporation
- The rate of evaporation increases on increasing the surface area of the liquid.
- The rate of evaporation increases with an increase in temperature.
- Decrease in the humidity increases the rate of evaporation.
- An increase in the wind speed increases the rate of evaporation
Effect of Change of Pressure
- Gases can be liquefied by applying pressure and reducing the temperature.
- When a high pressure is applied to a gas, it gets compressed and if the temperature is lowered, the gas is liquefied.
Plasma
- The state consists of super energetic and super excited particles. These particles are in the form of ionised gases.
- The fluorescent tube and neon sign bulbs consist of plasma.
