1 Classification of Metals, Conductors, and Semi-conductors
Classification of Metals, Conductors, and Semi-conductors
- Metals − Possess very low resistivity (or high conductivity)
- Semi-conductors − Possess resistivity or conductivity intermediate to metals and insulators
- Insulators − Possess high resistivity (or low conductivity)
Semi-conductors are of two types:
- Elemental semi-conductor − Example: Si and Ge
- Compound semi-conductor − Example: CdS, GaAs, CdSe, InP, etc.
Energy band diagram of metals or conductors

- Conduction band is partially filled and the valence band is partially empty or the conduction and valence band overlap.
- Due to overlap, electrons can easily move into the conduction band. This situation makes a large number of electrons available for electrical conduction.
- When the valence band is partially empty, electrons from their lower levels can move to higher levels making conduction possible.
Energy band diagram for insulators
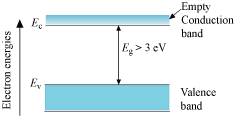
- Large band gap Eg exists. (Eg > 3 eV)
- Since there are no electrons in the conduction band, no electrical conduction is possible.
- The electron cannot be excited from the valence band to the conduction band by thermal excitation.
Energy band diagram for semi-conductors
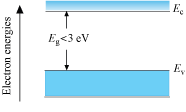
- Energy band gap Eg is small. (Eg < 3eV)
- At room temperature, some electrons from valence band cross the energy gap and enter the conduction band.
