2. Atomic Spectra
Atomic Spectra
- Each element emits a characteristic spectrum of radiation.
- In the excited state, the atoms emit radiations of a spectrum, which contains certain specific wavelengths only. This spectrum is termed as emission line spectrum and it consists of bright lines on a dark background.
- The spectrum emitted by atomic hydrogen is shown in the figure below.
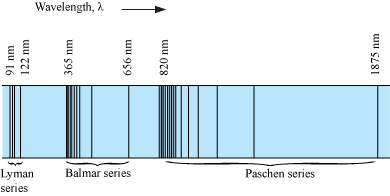
- Spectral Series
When the electron in a hydrogen atom jumps from higher energy level to the lower energy level, the difference of energies of the two energy levels is emitted as a radiation of particular wavelength. It is called a spectral line.
In H-atom, when an electron jumps from the orbit ni to orbit nf, the wavelength of the emitted radiation is given by,

Where,
R → Rydberg’s constant = 1.09678 ×107 m−1
For transition of the electron between two different energy levels, the spectral lines of different wavelengths are obtained. These spectral lines are found to fall into a number of spectral series as discussed below.
- Lyman series
For Lyman series, nf = 1 and ni = 2, 3, 4, …
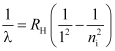
These spectral lines lie in ultraviolet region.
- Balmer series
For Balmer series, nf = 2 and ni = 3, 4, 5, …
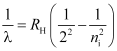
Where, ni = 3, 4, 5, …
These spectral lines lie in the visible region.
- Paschan series
For Paschan series, nf = 3 and ni = 4, 5, 6, …
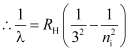
These spectral lines lie in the IR − region.
- Brackett series
For Brackett series, nf = 4 and ni = 5, 6, 7, …
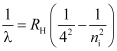
The spectral lines of this series lie in IR − region.
- Pfund series
For Pfund series, nf = 5 and ni = 6, 7, 8, …
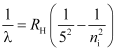
These series lie in the far infrared region.
