2 Size of Nucleus
Size of Nucleus
- It was found experimentally that the volume of a nucleus is proportional to its mass number (A).
- Let
R → Radius of the nucleus
∴Volume 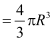

Where, R0 is a constant = 1.2 × 10−15 m is the range of nuclear force
- The density of nuclei of all the atoms is same as it is independent of mass number.
- Mass is another form of energy. One can convert mass-energy into other form of energy.
- Mass-energy equivalence relation is E = mc2
Where,
m → Mass
c → Speed of light
Nuclear Binding Energy
- The difference in mass of a nucleus and its constituents is called the mass defect.
- Binding energy of a nucleus is the energy with which nucleons are bound in the nucleus.
Expression for Binding Energy
- In a nucleus
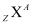 ,
,
Z = Number of protons
A = Number of protons + Number of neutrons
Let mp = Mass of a proton
mn = Mass of a neutron
mN = Mass of nucleus 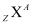
∴Mass defect,
Δm = [Zmp + (A − Z) mn − mN]
- Using Einstein’s mass-energy equivalence,
Binding energy = ΔmC2
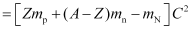
- Average binding energy per nucleon is given by the total binding energy divided by the mass number of the nucleus.
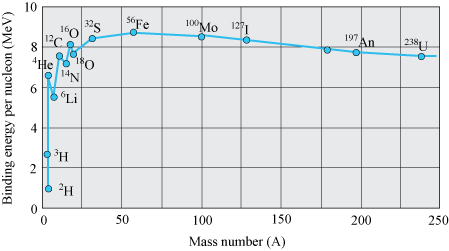
- Binding energy per nucleon is practically constant for mass number. (30 < A < 170)
- Binding energy per nucleon is lower for both light nuclei (A < 30) and heavy nuclei (A > 170).
Importance of Binding energy curve
- As we move from heavy nuclei region to the middle region of the plot, there is a gain in the overall binding energy and hence, in the release of energy. This indicates that energy can be released when a heavy nucleus breaks into roughly two equal fragments.
This process is called nuclear fission.
- When we move from lighter nuclei to heavier nuclei, there will be gain in the overall binding energy and release of energy. This is called nuclear fusion.
