2 Wave Theory of Light _ Energy Quantum of Radiation
Wave Theory of Light & Energy Quantum of Radiation
Einstein’s Photoelectric Equation
- Einstein explained the various laws of photoelectric emission on the basis of Planck’s quantum theory. According to Planck’s quantum theory, light radiations consist of small packets of energy called quanta. One quantum of light radiation is called a photon, which travels with the speed of light. The energy of a photon is given by,
E = hν
Where,
h − Planck’s constant
ν − Frequency of light
- Consider a photon of light frequency ν incident on a photosensitive metal surface. The energy of the photon (= hν) can be used in two ways:
(i) To liberate the electron from the metal surface (= Work function)
(ii) The rest of the energy of the photon is used in imparting the maximum kinetic energy kmax to the emitted photoelectrons.
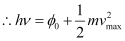
Where,
Φ0 − Work function of the metal
vmax − Maximum velocity of the emitted photoelectron
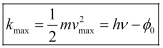
Where,
kmax − Maximum kinetic energy of the photoelectrons
