3. Bohr_s Model of Hydrogen Atom
Bohr’s Model of Hydrogen Atom
Bohr suggested a new model for the atom as Rutherford’s atom model was unstable. He introduced the concept of stationary orbits.
Postulates of Bohr’s Atom Model
- In a hydrogen atom, the negatively charged electron revolves in a circular orbit around the heavy positively charged nucleus. These are the stationary (orbits) states of the atom.
- The electrons revolve around the nucleus only in those orbits for which the angular momentum is the integral multiple of
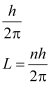
- Electron might make a transition from one of its specified non-radiating orbits to another of lower energy. When it does so, a photon is emitted having energy equal to the energy difference between the initial and final state.
hν = Ei − Ef
Ei > Ef,
Angular momentum is given by,
L = mvr
According to Bohr’s 2nd postulate,
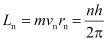
n → Principle quantum
vn → Speed of moving electron in the nth orbit
rn→ Radius of nthorbit
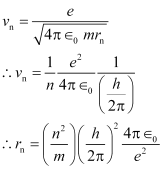
For n = 1 (innermost orbit),
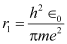
This is called Bohr radius, represented by the symbol a0.
Total energy,
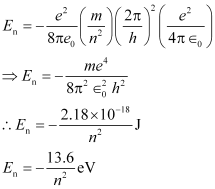
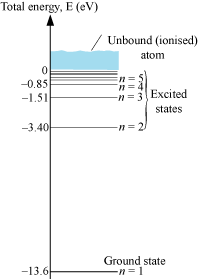
Energy level diagram for a hydrogen atom: