4. Line Spectra of Hydrogen Atom
Line Spectra of Hydrogen Atom
When an electron in a hydrogen atom jumps from the higher level to the lower energy level, the difference of energies of the two energy levels is emitted as a radiation of particular wavelength.
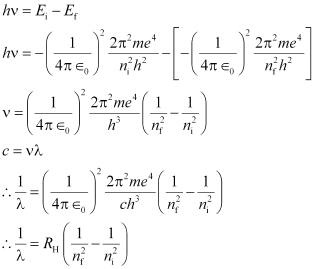
Where, 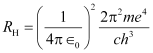
It is called Rydberg’s constant. Its value is 1.09678 × 107 m−1.
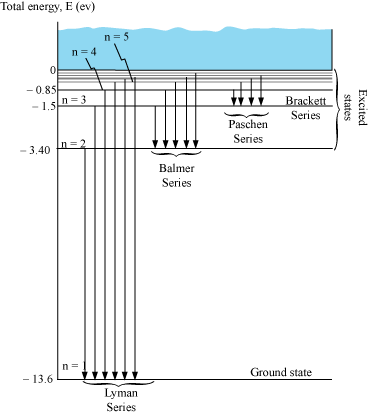
The different spectral series are as follows:
- Lyman series
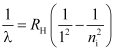
ni = 2, 3, 4 …
Lie in ultraviolet region
- Balmer series
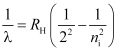
ni = 3, 4, 5 …
Lie in visible region
- Paschen series
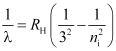
ni = 4, 5, 6 …
Lie in infra-red region
- Brackett series
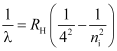
ni = 5, 6, 7 …
Lie in the infra-red region
- P fund series
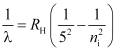
ni = 6, 7, 8 …
Lie in the far-infra red region
De Broglie’s Explanation of Bohr’s Second Postulate of Quantisation
- De-Broglie’s hypothesis that electron has a wavelength λ = h/mv gave an explanation for Bohr’s quantised orbits by bringing in the wave particle duality.
- Orbits correspond to circular standing waves in which the circumference of the orbits equal whole number of wavelength.
- Bohr’s model is applicable only to hydrogenic (single electron) atoms.
- It cannot be extended to even two electron atoms.
