4 Radioactivity
Radioactivity
There are three types of radioactive decay:
(i) α- decay
(ii) β- decay
(iii) γ- decay
Law of radioactive decay
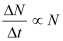
Where,
N = Number of nuclei in the sample
ΔN = Amount undergoing decay
Δt = Time
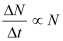
Where,
λ = Decay constant or disintegration constant
Δt = 0
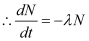
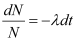
On integrating both sides, we get
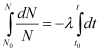
ln N − ln N0 = − λ (t − t0)
At t0 = 0,
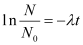
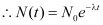
Decay rate (R) 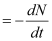
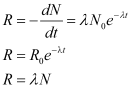
The total decay rate R of a sample is called the activity of that sample.
SI unit for activity is Becquerel.
1 Becquerel = 1 Bq = 1 decay per second
Half life
The half life of a radioactive substance is defined as the average time for which the nuclei of the atoms of the radioactive substance exist.
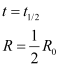
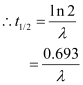
Average life or mean life (τ)

Alpha Decay
- Nucleus emits an alpha particle (a helium nucleus,
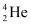 )
)
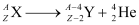
- Q value of an alpha decay:

Beta Decay
- Nucleus emits an electron or a positron
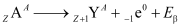
- In beta-minus decay,
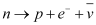
- In beta-plus decay,

Gamma Decay
When a nucleus is in an excited state, it can make a transition to a lower energy state by the emission of electromagnetic radiation. The stream of photons emitted by the nuclei is known as gamma ray, and the gamma ray has MeV energies.
