4 Wave Nature of Matter
Wave Nature of Matter
- Dual nature of matter − Radiation has dual nature i.e., it possesses properties of both wave and particle. Universe is composed of both radiation and matter. Therefore, de Broglie concluded that the moving material particle must also possess dual nature since nature loves symmetry.
- De Broglie hypothesis − According to de Broglie, a moving material particle sometimes acts as a wave and sometimes as a particle; or a wave is associated with moving material particle, which controls the particle in every respect. The wave associated with moving particle is called matter wave or de Broglie wave,
Where, m and v are the mass and velocity of the particle and h is Planck’s constant
- Derivation of de Broglie wavelength
According to Planck’s quantum theory, the energy of a photon of a radiation of frequency ν and wavelength λ is
E = hν …(i)
According to Einstein’s mass-energy relation,
E = mc2 …(ii)
From (i) and (ii), we obtain
hν = mc2
∴ 
Since each photon moves with the same velocity c, the momentum of photon, p = Mass × Velocity
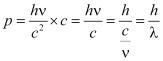
That is,

Equation (iv) is equally applicable to both the photons of radiation and other material particles.
