6 Magnetic Properties of Materials
Magnetic Properties of Materials
- Materials can be classified as diamagnetic, paramagnetic, or ferromagnetic on the basis of susceptibility (χ).
| Diamagnetic | Paramagnetic | Ferromagnetic |
| −1≤ χ≤ 0 | 0 < χ< ε | χ >> 1 |
| 0 ≤ μr < 1 | 1 < μr < 1 + ε | μr >> 1 |
| μ < μ0 | μ > μ0 | μ >> μ0 |
Here, ε is a small positive number introduced to quantify paramagnetic materials.
- Diamagnetism − Diamagnetic substances are those which have a tendency to move from stronger to the weaker part of the external magnetic field.

- When a bar of diamagnetic material is placed in an external magnetic field, the field lines are repelled or expelled and the field inside the material is reduced.
- Explanation of Diamagnetism − Diamagnetic substances are the ones in which resultant magnetic moment in an atom is zero.
When magnetic field is applied, those electrons having orbital magnetic moment in the same direction slow down and those in the opposite direction speed up. This happens due to induced current in accordance with Lenz law.
Thus, the substance develops a net magnetic moment in direction opposite to that of the applied field and hence, repels.
- Examples of Diamagnetic materials − Bismuth copper, lead, silicon, nitrogen (at STP), water, and sodium chloride
- Meissner effect − Superconductors exhibit perfect diamagnetism. A superconductor repels a magnet and is repelled by the magnet. This phenomenon of perfect diamagnetism in superconductors is called the Meissner effect.
- Paramagnetism − Substances that have the tendency to move from a region of weak magnetic field to strong magnetic field i.e, they get weakly attracted to a magnet
- Explanation of paramagnetism − The atoms of a paramagnetic material possess a permanent magnetic dipole moment of their own. On account of the ceaseless random motion of the atoms, no net magnetisation is seen. In the presence of an external field B0, which is strong enough, and at low temperatures, the individual atomic dipole moment can be made to align and point in the same direction as B0.

The field lines get concentrated inside the material and the field inside is enhanced.
- Examples of paramagnetic materials − Aluminium, sodium, calcium, oxygen (at STP), and copper chloride
- Curie’s law − Magnetisation of a paramagnetic material is inversely proportional to the absolute temperature T.
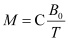
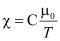
Where, C is called Curie’s constant
- Ferromagnetism − Substances which get strongly magnetised when placed in an external magnetic field
- Explanation of Ferromagnetism − The atoms in a ferromagnetic material possess a dipole moment aligned in a common direction over a macroscopic volume called domain. Each domain has a net magnetisation.
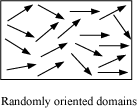
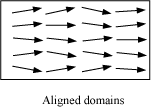
When we apply an external magnetic field B0, the domains orient themselves in the direction of B0 and simultaneously the domain grows in size.
- The ferromagnetic property depends on temperature. At high temperature, a ferromagnet becomes a paramagnet. The temperature of transition from ferromagnetic to paramagnetic is called the Curie temperature (TC). The susceptibility in the paramagnetic phase is described by,

- Hysterisis
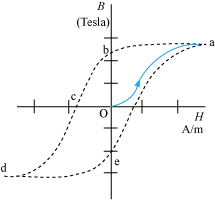
The above graph shows the behaviour of the material as we take it through one cycle of magnetisation.
An unmagnetised sample is placed in a solenoid and current through the solenoid is increased. The magnetic field B in the material rises and saturates as depicted in the curve Oa. Next, H is decreased and reduced to zero.
At H = 0, B ≠ 0 (curve ab)
The value of B at H = 0 is called retentivity.
Now, the current in the solenoid is reversed and slowly increased. Certain domains are flipped until the net field inside stands nullified. This is represented by the curve bc.
The value of H at c is called coercivity. As the reversed current is increased in magnitude, we once again obtain saturation (curve cd).
Now, the current is reduced (curve de) and reversed (curve ea). The cycle repeats itself. For a given value of H, B is not unique, but depends on previous history of the sample. This phenomenon is called hysterisis.
