1. Amorphous and Crystalline Solids
- Based on the nature of the order of arrangement of the constituent particles, solids are classified as amorphous and crystalline.
- Differences between amorphous and crystalline solids are listed in the given table.
| Amorphous solids | Crystalline solids | ||
| 1 | Have irregular shape | 1 | Have definite characteristic geometrical shape |
| 2 | Have only short-range order in the arrangement of constituent particles | 2 | Have long-range order in the arrangement of constituent particles |
| 3 | Gradually soften over a range of temperature | 3 | Have sharp and characteristic melting point |
| 4 | When cut with a sharp-edged tool, they cut into two pieces with irregular shapes | 4 | When cut with a sharp-edged tool, they split into two pieces with plain and smooth newly generated surfaces. |
| 5 | Do not have definite heat of fusion | 5 | Have definite and characteristic heat of fusion |
| 6 | Isotropic in nature | 6 | Anisotropic in nature |
| 7 | Pseudo solids or super-cooled liquids | 7 | True solids |
Classification of Crystalline Solids
- Based on the nature of intermolecular forces, crystalline solids are classified into four categories −
-
- Molecular solids
- Ionic solids
- Metallic solids
- Covalent solids
- Molecular solids
- Constituent particles are molecules
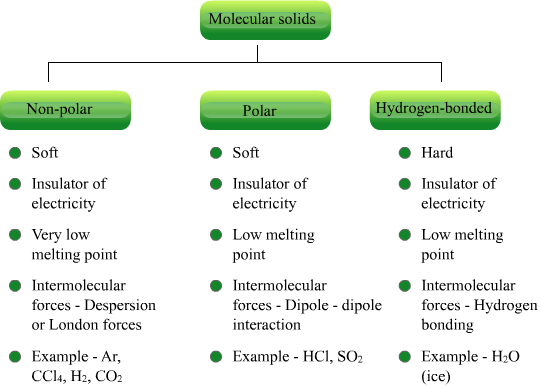
- Constituent particles are molecules
- Ionic solids
- Constituent particles are ions
- Hard but brittle
- Insulators of electricity in solid state, but conductors in molten state and in aqueous solution
- High melting point
- Attractive forces are Coulombic or electrostatic
- Example − NaCl, MgO, ZnS
- Metallic solids
- In metallic solids, positive ions are surrounded and are held together in a sea of delocalised electrons.
- Hard but malleable and ductile
- Conductors of electricity in solid state as well as molten state
- Fairly high melting point
- Particles are held by metallic bonding
- Example − Fe, Cu, Mg
- Covalent or network solids
- Constituent particles are atoms
- Hard (except graphite, which is soft)
- Insulators of electricity (except graphite, which is a conductor of electricity)
- Very low melting point
- Particles are held by covalent bonding
- Example − SiO2 (quartz), SiC, diamond, graphite
| Add to your knowledge |
| The property by virtue of which two or more crystalline solids having similar chemical composition exist in the same crystalline form is called isomorphism. For example: Na3PO4.
The property by virtue of which a particular substance exists in more than one crystalline form is called polymorphism. For example: existence of calcium carbonate in two crystalline forms called calcite and aragonite. |

1 Comment
Amazing 🤩 Sir